Abstract and Introduction
Abstract
Background & Aims: Sequential therapy posed a high risk of emergence of multidrug resistance and presented a management issue in chronic hepatitis B (CHB) treatment. We evaluated the antiviral efficacy and safety of entecavir (ETV) plus tenofovir (TDF) combination therapy in multidrug-resistant (MDR) CHB patients.
Methods: In this prospective, multicentre study, MDR CHB patients, defined as measurable serum HBV DNA (≥60 IU/ml) while on any rescue treatment regimen for at least 24 weeks and the presence of documented prior genotypic resistance to both nucleoside analogue(s) and nucleotide analogue, were treated with ETV 1.0 mg and TDF 300 mg combination therapy for 48 weeks.
Results: A total of 64 eligible patients who had previously failed to a median three lines of antiviral therapy (range, 2–6) were included. At baseline, median age was 47.0 years, 89.1% were HBeAg(+), and median HBV DNA was 4.24 (range, 2.11–6.73) log10 IU/ml. By week 4, 12, 24 and 48, 15/64 (23.4%), 36/64 (56.3%), 43/64 (67.2%) and 55/64 (85.9%) patients achieved a HBV DNA <60 IU/ml respectively. The mean reduction of HBV DNA from baseline to 4 and 48 weeks was 1.23 log10 IU/ml and 2.38 log10 IU/ml respectively. Although five patients experienced virological breakthrough, all were transient and no resistant mutation to TDF or novel mutation was detected in any patients.
Conclusions: In difficult-to-treat MDR CHB patients with a high exposure to multiple antiviral drugs, ETV plus TDF combination therapy can provide a very high rate of viral suppression through 48 weeks of treatment.
Introduction
Although nucleos(t)ide analogues (NAs) have revolutionized the treatment of chronic hepatitis B (CHB), chronic hepatitis B virus (HBV) infection remains a global health issue, affecting an estimated 300–400 million people worldwide and accounting for more than 500 000 deaths annually.[1,2] The main goal of therapy for CHB is a sustained viral suppression with undetectable HBV DNA, thereby preventing the development of chronic liver disease.[3–5] HBV infection cannot be completely eradicated by currently available antiviral drugs, because of the persistence of a stable intrahepatic reservoir of covalently closed circular DNA. Hence, with NAs, long-term treatment is often required to maintain the response to treatment.
However, as the duration of NA treatment becomes prolonged, the risk of development of drug-resistant mutants increases, especially with less potent and lower genetic barrier drugs, such as lamivudine (LAM) and adefovir (ADV), which were prescribed extensively in the era of the early generation NAs. Drug resistance is a well-known cause of therapeutic failure. Also, to date, many patients have been treated with sequential monotherapy after the development of viral and clinical breakthrough. Such sequential monotherapy can promote selection for multidrug-resistant (MDR) strains, especially when patients are treated sequentially with drugs with overlapping resistance profiles, such as with LAM followed by ADV or LAM followed by entecavir (ETV) or ADV followed by tenofovir disoproxil fumarate (TDF).[6] As a result, many patients have experienced treatment failure with multiple NAs, which has been a major challenge for clinicians. The management of antiviral resistance should develop based on a better understanding of the mechanisms of resistance and experience learned from managing patients with LAM resistance.
ETV and TDF are high-potency inhibitors of HBV polymerase, and current international guidelines recommend that ETV and TDF should be used as first-line NAs for naïve CHB patients to prevent the development of drug resistance during long-term treatment.[3–5] Both drugs achieved high rates of HBV DNA suppression and ALT normalization and demonstrated a favourable safety profile in long-term studies.[7,8]
In cases of resistance, combination therapy with a nucleoside analogue and a nucleotide analogue has the theoretical advantage of offering synergistic viral suppression and a higher genetic barrier to resistance formation.[6,9] Recently, increasing evidence suggests that combination therapy can effectively suppress viral replication and significantly delay or prevent the emergence of drug resistance.
The combination of ETV and TDF should be a promising salvage therapeutic option in management of refractory CHB patients who have failed several lines of therapy. However, there are limited data on the efficacy of ETV-TDF combination therapy in MDR CHB patients. There have been few retrospective studies on the efficacy of ETV-TDF combination therapy for the treatment of CHB patients who have failed multiple lines of treatment.[10,11]
In this study, we present 48-week results from a prospective evaluation of the efficacy and safety of ETV-TDF combination therapy for CHB patients who have developed MDR strains after experiencing multiple NA failures.
Patients and Methods
Study Design
This was a prospective, phase 3b, multicentre, 48-week, open-label ETV 1.0 mg-TDF clinical study (the 'ESTEEM' study; NCT01594905) conducted in patients with MDR CHB, defined as measurable serum HBV DNA (≥60 IU/ml) while on any rescue treatment regimen for at least 24 weeks, with exposure to both nucleoside analogue(s) (LAM, telbivudine, clevudine, ETV) and nucleotide analogue (ADV) at any previous time, and the presence of documented historic or current genotypic resistance to both nucleoside analogue(s) and nucleotide analogue. Patients received the fixed-dose combination ETV 1.0 mg and TDF 300 mg once daily for 48 weeks. The first patient was screened in August 2012, and the last patient's follow-up occurred in May 2014. Written informed consent was obtained from all study patients. The protocol was approved by the institutional review board (Severance Hospital IRB).
Patients
Adult patients were considered for the study if they satisfied all of the following inclusion criteria: HBsAg positivity for more than 6 months before screening, the presence of persistent viraemia, defined as serum HBV DNA levels higher than 60 IU/ml after any rescue therapy for at least 24 weeks, and the previous experience of documented genotypic resistance to both nucleoside analogue(s) and a nucleotide analogue at any time before screening. Patients had well-preserved liver function, and no history of ascites, variceal bleeding or encephalopathy. Additionally, patients were required to have a creatinine clearance of >50 ml/min. Prior interferon was permitted if it had been discontinued at least 6 months before screening.
Patients were excluded if they were coinfected with hepatitis C/D, or human immunodeficiency virus, had a history of hepatocellular carcinoma, were receiving concurrent systemic immunosuppressive agents, had a history of non-alcoholic fatty liver disease or alcohol/substance abuse, were diagnosed with a malignant tumour and had been receiving chemotherapy, or had a history of liver transplantation. Those with alanine aminotransferase (ALT) levels at screening >15 times upper limit of normal range (ULN: 30 IU/L for males and 19 IU/L for females), pregnant or breastfeeding females and patients with serious concurrent medical conditions were also excluded.
There was no interruption in treatment with previous NA treatment before starting the study drugs.
Study Objectives and Endpoints
The primary efficacy end point was the proportion of subjects who achieved a complete virological response (CVR, HBV DNA <60 IU/ml) by real-time PCR at week 48 after ETV 1.0 mg plus TDF combination therapy. The secondary efficacy end points included the proportion of patients with HBV DNA <12 IU/ml at week 48, ALT normalization (≤1 times ULN), HBeAg loss and seroconversion (HBeAg-positive patients only), HBsAg loss and changes in serum HBV DNA concentrations over time.
Follow-up and Monitoring
Patients were evaluated at baseline and at weeks 4, 12, 24, 36 and 48. At each visit, patients were evaluated for clinical adverse events and compliance with study medications. Blood was taken for investigations including liver and renal function tests, complete blood counts, calcium, phosphate and HBV DNA levels. HBV serology including HBsAg, anti-HBs, HBeAg and anti-HBe, tested with enzyme-linked immunoassays (Abbott Diagnostics, Abbott Park, IL, USA), were performed at baseline, week 24 and week 48. HBV DNA levels were measured by quantitative PCR assay (Amplicor HBV Monitor Test; Roche Diagnostics, Basel, Switzerland) with the limit of quantification (LOQ) = 60 IU/ml and lower limit of detection (LLD) = 12 IU/ml, in a central laboratory. Because almost all HBV patients in Korea (>95%) are infected with HBV genotype C,[12] HBV genotype was not determined. Antiviral drug resistance mutations were determined using multiplex restriction fragment mass polymorphism (RFMP), as described previously,[13] for all patients at baseline and for viraemic patients (HBV DNA >60 IU/ml) at any follow-up time. The amino acid substitutions conferring resistance to LAM (rtM204 and rtL180), ADV (rtA181 and rtN236), ETV (rtI169, rtT184, rtS202 and rtM250) and TDF (rtA194) were analysed.
Safety Analyses
Safety and tolerability were evaluated by the occurrence of adverse events (AEs), serious AEs, laboratory abnormalities, discontinuation of the study drugs due to AEs or death during the entire study period. ALT flares were defined as ALT levels >2 times baseline and >10 times ULN. Potential nephrotoxicity (including an increase in serum creatinine of at least 0.5 mg/dl above baseline, a decrease in serum phosphorus <2 mg/dl or creatinine clearance decrease to <50 ml/min) parameters were monitored at each visit.[14]
Statistical Analysis
Efficacy and safety analyses included all patients who received at least one dose of study medication. A sample size of 64 patients was considered to be adequate for the study, based on the assumption of a proportion with CVR for TDF rescue therapy at week 48[15] and an expected increase in CVR of 25% for ETV 1 mg-TDF rescue therapy at week 48 with a power of 80%, and allowing for a dropout rate of 10%. Patients who discontinued study medication before week 48 for any reason were considered treatment failures.
All continuous variables are summarized with descriptive statistics, and categorical data are presented as numbers (%). Between-group comparisons of continuous or categorical variables were conducted using a t-test, χ2 test or Fisher's exact test, as appropriate. Serum HBV DNA levels are expressed in logarithms. The log value was defined as 1.78 when below the LOQ value. A P value <0.05 was considered to indicate statistical significance.
Results
Study Population Of the 73 patients screened in this study, 64 were treated with ETV-TDF. Of them, 63 patients completed 48 weeks of treatment, while one was lost to follow-up at 48 week. All patients were confirmed to be adherent to antiviral therapy. The baseline demographic, clinical and laboratory characteristics of 64 patients are summarized in Table 1. All patients had previously been exposed to both nucleoside and nucleotide analogues and were confirmed to be infected with HBV with genotypic resistance to both NAs. However, no patient had prior exposure to TDF. Two patients had a history of previous pegylated interferon experience and 41 (64.1%) had prior exposure to ETV. As a first-line antiviral therapy, 58 (90.6%) patients had been treated with LAM, four with ETV and two patients with ADV. In total, 34 (53.1%) patients had confirmed ETV resistance by medical history. Last treatment regimens before this study were LAM/ADV (n = 12), LdT/ADV (n = 15), ETV/ADV (n = 23), ADV (n = 9) and ETV (n = 5). In terms of the baseline resistance profile, 62 (96.9%) showed antiviral resistance to at least one class of NAs: two patients had wild-type HBV strains and seven were infected with HBV strains with amino acid substitutions conferring resistance to LAM and ADV, 12 to LAM and ETV, 7 to ETV and ADV and 9 to LAM, ETV and ADV. In patients with ADV resistance, 42 and 19 were found to harbour the rtA181V/T and rtN236T mutations respectively.
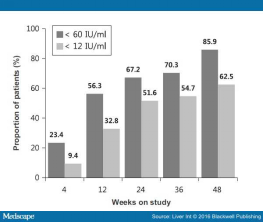
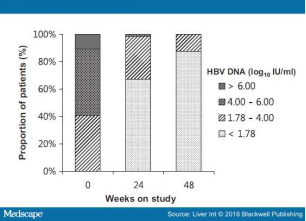
Virological Response Of the study population, 55 of 64 (85.9%) achieved CVR at week 48 of ETV 1 mg-TDF combination therapy (Fig. 1). More than half of the patients (56.3%) had HBV DNA <60 IU/ml at week 12, rising to 67.2% at week 24. Regarding the lower limit of detection of HBV DNA, 12 IU/ml, the response rates were 32.8% at week 12, 51.6% at week 24 and 62.5% at week 48. The HBV viral load continued to fall throughout the course of the study. The overall mean HBV DNA concentrations were 3.06 (SD 1.02), 2.34 (SD 0.77), 2.12 (SD 0.60) and 1.91 (SD 0.40) log10 IU/ml at weeks 4, 12, 24 and 48 respectively. Almost all patients experienced an initial rapid reduction in viral load and then an additional steady reduction. With the exception of two patients, all had a fall of >2 log10 IU/ml in HBV DNA by 12 weeks of therapy, and this was achieved at 24 weeks in the remaining two patients. Baseline HBV DNA level had no impact on virological response in this study (data not shown). The distribution of HBV DNA levels through week 48 is shown in Fig. 2.
Virological response according to the documented baseline resistance profile is shown in Table 2 . Median baseline HBV DNA levels were significantly lower in patients with genotypic resistance to LAM and ADV, compared with the remaining patients in the study (P < 0.001). Through 48 weeks of therapy, there was no significant difference in CVR according to the baseline resistance profile, although patients with triple genotypic resistance to LAM, ADV and ETV had a slightly smaller proportion of virological responses at week 48. Of the 42 patients with baseline ADV-associated mutations, 36 (85.7%) achieved a CVR by week 48. Among patients harbouring ADV-associated mutations, according to the presence or absence of baseline rtN236T mutations, the viral kinetic curves were nearly identical for both groups (Fig. S1). Of 19 patients harbouring baseline rtN236T mutations, 17 (89.5%) achieved CVR to ETV-TDF combination therapy, whereas 20 of the remaining 23 patients (87.0%) achieved CVR at week 48.
Resistance surveillance was performed on all samples at baseline and patients with detectable serum HBV DNA. During the study period, five patients experienced virological breakthrough. However, all breakthroughs were transient and HBV DNA levels subsequently declined with continued treatment. Eight patients without CVR at 48 weeks qualified for genotypic resistance analysis by RFMP (Table S1). Overall, no resistant mutation to TDF or novel mutation was detected in any patient.
Biochemical and Serological Responses
Biochemical and serological response are summarized in Table 3. The percentages of patients with normal ALT levels (<1 × ULN) at weeks 24 and 48 were 45.3% (29/64) and 50.0% (32/64) respectively. Mean serum ALT levels decreased progressively from 39.7 (SD 54.0) IU/L at baseline to 30.6 (SD 15.5) IU/L at week 48 (not significant).
Of the 57 patients who were HBeAg-positive at baseline, two became HBeAg negative after 24 weeks of ETV-TDF combination therapy. No patient achieved HBeAg seroconversion or HBsAg loss at week 48.
Safety and Side Effects
The ETV-TDF combination therapy was well tolerated, and no clinically significant AE was noted during the study period. No patient required dose interruption or discontinuation of treatment due to an AE. The most frequent AEs were nausea, dyspepsia and chest discomfort reported in 4.7%, 3.1% and 3.1% of patients respectively. On-treatment hepatic flare was reported in one HBeAg-positive patient. It occurred at 4 weeks of treatment and might have been associated with a decline in the HBV DNA level and subsequent HBeAg loss. No other liver-related events occurred during the study period.
Only one patient experienced deterioration of renal function; the serum creatinine level increased by >0.5 mg/dl during combination therapy, which resolved without drug modification or treatment interruption. Creatinine levels in this patient increased from 0.60 mg/dl at baseline to 1.32 mg/dl at 36 weeks but decreased to 0.58 mg/dl at 48 weeks. No patient showed the other renal endpoints. Median serum creatinine level was 0.87 (range, 0.38–1.30) mg/dl at baseline and 0.90 (range, 0.57–1.28) mg/dl at the end of the study (not significant).
Discussion
Over past decades, NAs that potently inhibit the viral polymerase have been used widely in the treatment of chronic HBV infection. Until recently, many patients worldwide began antiviral treatment with less potent drugs, such as LAM or ADV, which also have a low genetic barrier to resistance. Exposure to multiple NAs with low barriers to resistance increases the risk of multidrug resistance. Indeed, the emergence of viral resistance during treatment is still an important clinical issue for antiviral therapy. In this prospective 48-week single-arm clinical trial, we demonstrated that ETV 1.0 mg-TDF combination therapy was highly effective in CHB patients who had developed MDR strains after experiencing resistance to multiple NAs, with 85.9% of patients achieving the primary study endpoint (HBV DNA <60 IU/ml at week 48). Furthermore, 62.5% of patients achieved HBV DNA <12 IU/ml by week 48. Even in patients who did not meet the primary endpoint, serum HBV DNA levels decreased gradually during the treatment period and were at very low levels by week 48. Baseline viral load, exposure to several different groups of NAs, the presence of resistance mutations at the time of treatment initiation and/or historic genotypic resistance did not affect treatment efficacy in this study. This study revealed that the great majority of patients with MDR CHB showed a definite therapeutic benefit of ETV-TDF combination therapy. The results that we observed were favourably comparable with those reported in retrospective studies of ETV-TDF combination therapy in CHB patients with previous multiple NA treatment failure.[10,11] Our experience should serve to support clinical evidence of ETV-TDF combination therapy in patients with MDR CHB.
A crucial concept in the management of antiviral resistance is that add-on therapy rather than switching monotherapy appears to be a superior approach with regard to preventing subsequent multidrug resistance.[6,9,16] This concept originally arose from issues in the management of LAM-resistant patients. While no difference in rates of response or virological breakthrough was evident with treatment for 12 months,[17] long-duration treatment showed the superiority of combination therapy over adefovir monotherapy in preventing adefovir resistance.[18] Also, even though ETV has higher potency and a higher genetic barrier to resistance, the cumulative probabilities of genotypic resistance to ETV and virological breakthrough increased to 51% and 43%, respectively, in LAM-refractory patients who were switched to ETV monotherapy due to potential cross-resistance.[7] However, in the beginning, LAM resistance was commonly managed by switching to ADV or ETV, which resulted in a high incidence of multidrug resistance and earlier international guidelines, which were based on insufficient clinical evidence, recommended these rescue treatment strategies. In cases of antiviral resistance, an appropriate rescue therapy should be launched earlier and should have the most effective antiviral effect and minimize the risk for subsequent development of resistance to the new agent. To date, several studies have reported the efficacy of TDF alone or combined with nucleoside analogues for the treatment of CHB refractory to NAs. Fung et al. compared TDF monotherapy with combined TDF and emtricitabine therapy in CHB patients with genotypic LMV resistance and concluded that the antiviral effect of TDF monotherapy was satisfactory and comparable to that of combined TDF and emtricitabine therapy.[19] Berg et al. reported that TDF monotherapy and combined TDF/emtricitabine therapy had similar efficacy in ADV incomplete responders.[14] In several studies, TDF-based combination therapy has shown no benefit over TDF alone, at least during relatively short follow-up durations. Moreover, recent two randomized-controlled and multicentre trials showed that TDF monorescue therapy was not inferior to ETV-TDF combination therapy in patients with ADV resistance or ETV resistance.[20,21] However, it should be emphasized that although TDF monotherapy was as effective as TDF-based combination therapy initially, there are still concerns over the potential emergence of resistance to TDF, especially in MDR CHB patients with exposure to multiple alternating NAs. An in vitro susceptibility study showed that HBV clones harbouring rtN236T or rtA181V/T showed decreased susceptibility to TDF, of about four-fold, and the double mutation resulted in a reduction in TDF sensitivity of 7- to 10-fold.[22] Among in vivo studies, Tan et al. reported that in patients who had adefovir-resistant mutations, the response to TDF would be slower or suboptimal, suggesting that combination therapy with nucleoside analogue such as emtricitabine might be a more effective rescue therapy for patients with ADV resistance.[23] Another recent study analysed the antiviral efficacy of TDF monotherapy or TDF-LAM combination therapy in patients with prior failure to both LAM and ADV.[15] At 48 and 96 weeks, 46% and 64% patients achieved undetectable HBV DNA. Although no association between response and baseline ADV resistance was observed, individual patient plots showed that the presence of ADV resistance affected the subsequent virological response to TDF rescue therapy, even though combined with LAM. These results suggest that adding a low potency drug, such as LAM, to TDF may not be sufficient to delay the selection of MDR mutations. Long-term close monitoring of these patients may be required. Treatment strategies for difficult-to-treat MDR CHB patients should focus on preventing additional resistance by suppressing viral replication as early and completely as possible. The combination of ETV plus TDF, the most potent drugs with high genetic barriers and compensatory cross-resistance profiles, may be a more effective rescue therapy for MDR CHB patients with previous multiple NA failures. Importantly, TDF monotherapy may be less effective in the presence of additional ETV resistance mutations on ADV resistance.[10,11,24]
There are practical issues that argue against the use of TDF-based combination therapy. Although it may not be appropriate to apply a treatment concept based on data from drugs with low potency to highly potent, high genetic barrier drugs, the benefit of TDF-based combination therapy in MDR CHB patients will minimize the development of resistance to TDF even though combination therapy may show no additive/synergistic antiviral effects. Further research and additional data with long-term clinical and molecular virology study are needed to assess this issue in the NA era.
The most challenging issue in the treatment of CHB is how long therapy should be continued. Moreover, little is known about the optimum period of combination therapy. Long-term combination therapy may have undesirable or harmful effects, such as higher treatment costs, possibly lower adherence rates and safety concerns. Recently, an adapting step-down strategy, switching from combination therapy to monotherapy in virologically suppressed CHB patients with stable liver disease, was introduced.[10,25] This adapting step-down strategy may reduce the cost burden and the risk of potentially harmful effects of combination therapy.
To our knowledge, we have reported one of the largest prospective clinical trials of the efficacy of ETV-TDF combination therapy for difficult-to-treat MDR CHB patients. There have been several retrospective chart reviews for this combination therapy previously. Also, a distinguishing feature of this study is that we used a well-defined study population. All of the patients had histories of exposure to both nucleoside analogue(s) and a nucleotide analogue and confirmed historic genotypic resistance in both different mutational pathways, which is typical for a MDR CHB patient population. All of the patients were already confirmed to be infected with HBV with pre-existing ADF resistance. Once drug resistant mutants have been selected, they do not disappear but are retained persistently. We also analysed drug resistance mutations for all patients at baseline and for viraemic patients at any follow-up time by RFMP assay, which had been well correlated with clonal analysis.[13]
A major limitation of this study is the single-arm design, meaning the absence of a TDF monotherapy arm. However, at the time of study inception, there were limited clinical and published data about the effectiveness of TDF-based rescue therapy in patients with antiviral resistance, especially MDR mutants. In these patients, the efficacy of TDF monotherapy may be expected to be reduced by a history of exposure to multiple drugs and by the presence of ADV resistance mutations. Because of general concerns about the probability of newly developing TDF resistance in MDR CHB patients, it was felt that mandating initiation of ETV 1.0 mg-TDF combination therapy would avoid the risk of virological failure in those patients.
In summary, our data demonstrate that a combination of two potent drugs, TDF and ETV, is an effective therapy for difficult-to-treat MDR CHB patients with previous multiple NA failures, where it induced high CVR in more than 80% of patients by 48 weeks. The antiviral efficacy of ETV-TDF combination therapy was not influenced by the type of prior NAs, the presence of prior or baseline resistance mutations and baseline HBV DNA level. No resistance to TDF was detected in this study population. Future investigation of TDF-based therapy in prospective randomized long-term studies focusing on the best management strategy for CHB patients with MDR mutations is warranted.